How to run the Type a Known Species workflow
To run the workflow, go to:
Typing and Epidemiology (![]() ) | Workflows (
) | Workflows (![]() ) | Type a Known Species (
) | Type a Known Species (![]() )
)
- Specify the sample(s) or folder(s) of samples you would like to type (figure 24.46) and click Next. Remember that if you select several items, they will be run as batch units.
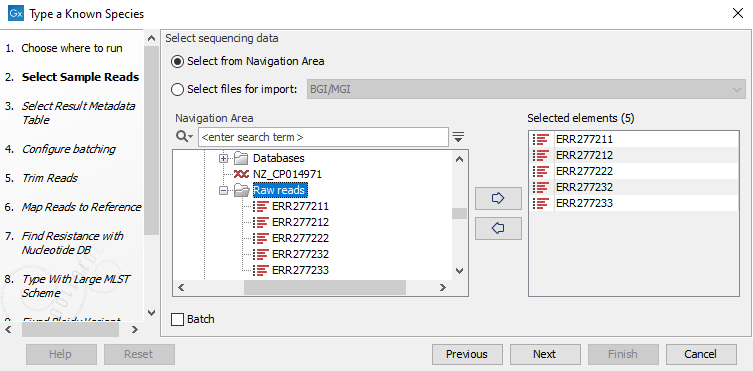
Figure 10.25: Select the reads from the sample(s) you would like to type. - Specify the Result Metadata Table you want to add your results to (figure 10.26) and click Next.
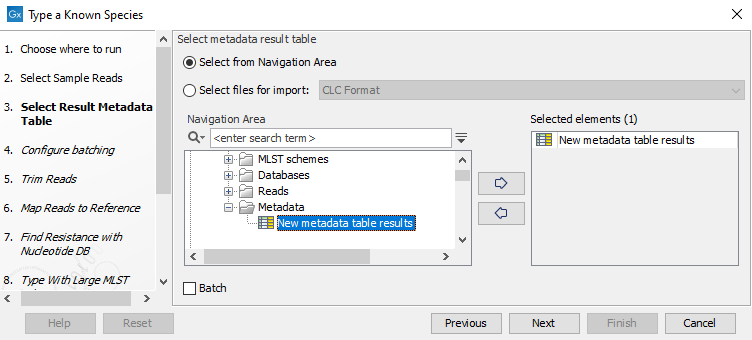
Figure 10.26: Select the metadata table you would like to use. - Define batch units using organisation of input data to create one run per input or use a metadata table to define batch units. Click Next.
- The next wizard window gives you an overview of the samples present in the selected folder(s). Choose which of these samples you want to analyze in case you are not interested in analyzing all the samples from a particular folder (figure 24.48).
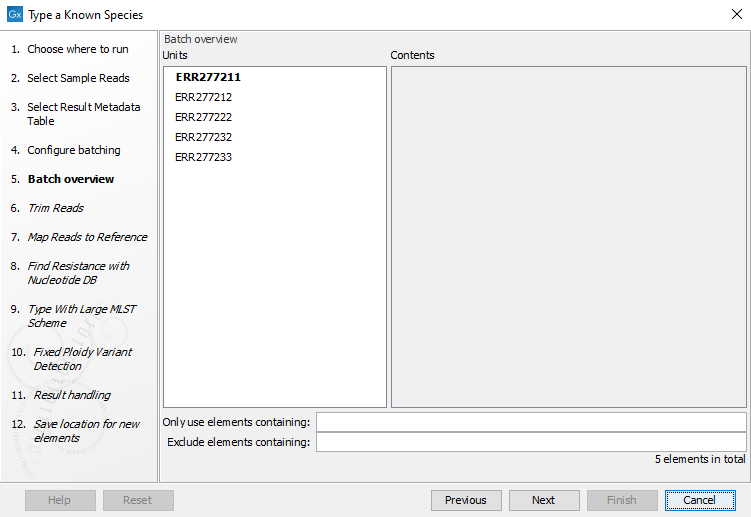
Figure 10.27: Choose which of the samples present in the selected folder(s) you want to analyze. - You can specify a trim adapter list and set up parameters if you would like to trim your sequences from adapters. Specifying a trim adapter list is optional but recommended to ensure the highest quality data for your typing analysis (figure 10.28). Learn about trim adapter lists at http://resources.qiagenbioinformatics.com/manuals/clcgenomicsworkbench/current/index.php?manual=Trim_adapter_list.html.
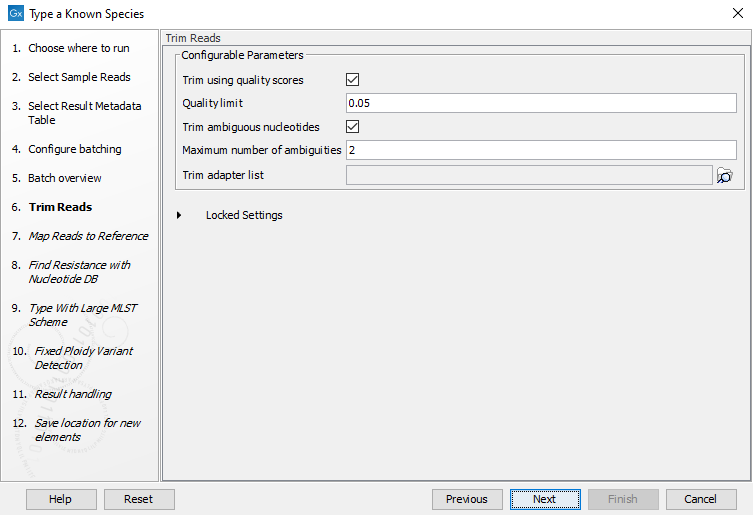
Figure 10.28: You can choose to trim adapter sequences from your sequencing reads.The parameters that can be set are:
- Trim ambiguous nucleotides: if checked, this option trims the sequence ends based on the presence of ambiguous nucleotides (typically N).
- Maximum number of ambiguities: defines the maximal number of ambiguous nucleotides allowed in the sequence after trimming.
- Trim using quality scores: if checked, and if the sequence files contain quality scores from a base-caller algorithm, this information can be used for trimming sequence ends.
- Quality limit: defines the minimal value of the Phred score for which bases will not be trimmed.
- Choose the species reference to be used by the Map reads to reference tool (figure 10.29). Click Next.
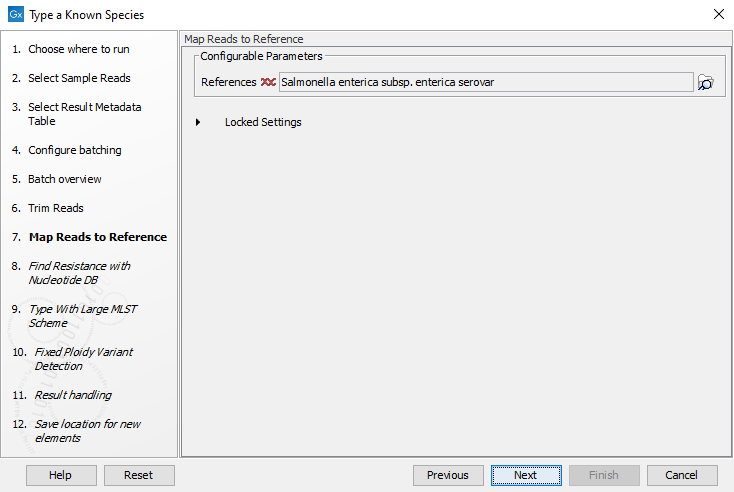
Figure 10.29: Specify the reference for the Map reads to reference tool. - Specify the resistance database (figure 10.30) and set the parameters for the Find Resistance with Nucleotide DB tool.
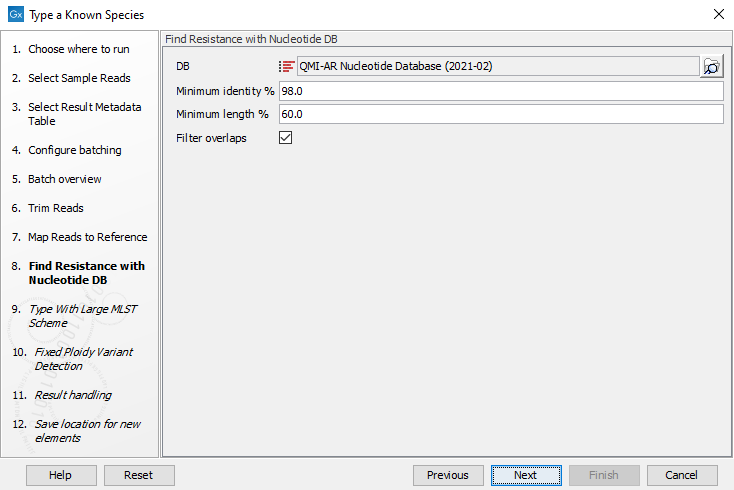
Figure 10.30: Specify the resistance database to be used for the Find Resistance with Nucleotide DB tool.The parameters that can be set are:
- Minimum Identity %: is the threshold for the minimum percentage of nucleotides that are identical between the best matching resistance gene in the database and the corresponding sequence in the genome.
- Minimum Length %: reflect the percentage of the total resistance gene length that a sequence must overlap a resistance gene to count as a hit for that gene. Here represented as a percentage of the total resistance gene length.
- Filter overlaps: will perform extra filtering of results per contig, where one hit is contained by the other with a preference for the hit with the higher number of aligned nucleotides (length * identity).
- Specify the parameters for the Type with Large MLST Scheme tool (figure 10.31).
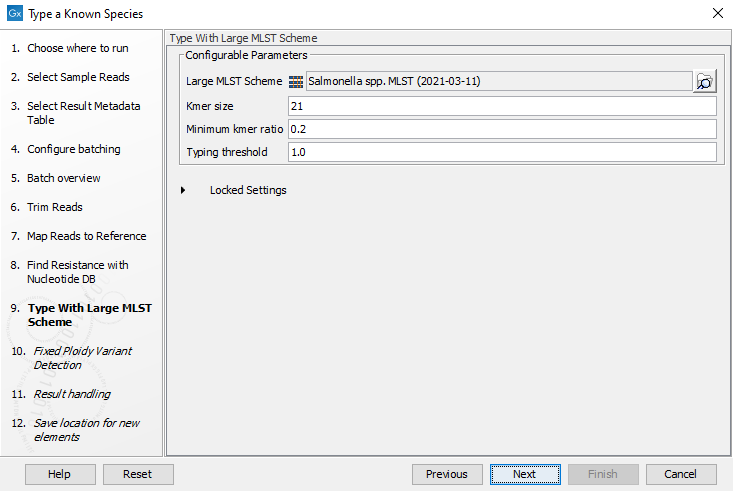
Figure 10.31: Specify the parameters for MLST typing.The parameters that can be set are:
- Kmer size: determines the number of nucleotides in the kmer - raising this setting might increase specificity at the cost of some sensitivity.
- Typing threshold: determines how many of the kmers in a sequence type that needs to be identified before a typing is considered conclusive. The default setting of 1.0 means that all kmers in all alleles must be matched.
- Minimum kmer ratio: the minimum kmer ratio of the least occurring kmer and the average kmer hit count. If an allele scores higher than this threshold it is classified as a high-confidence call.
Click Next.
- Specify the parameters for the Fixed Ploidy Variant Detection tool (figure 10.32) before clicking Next.
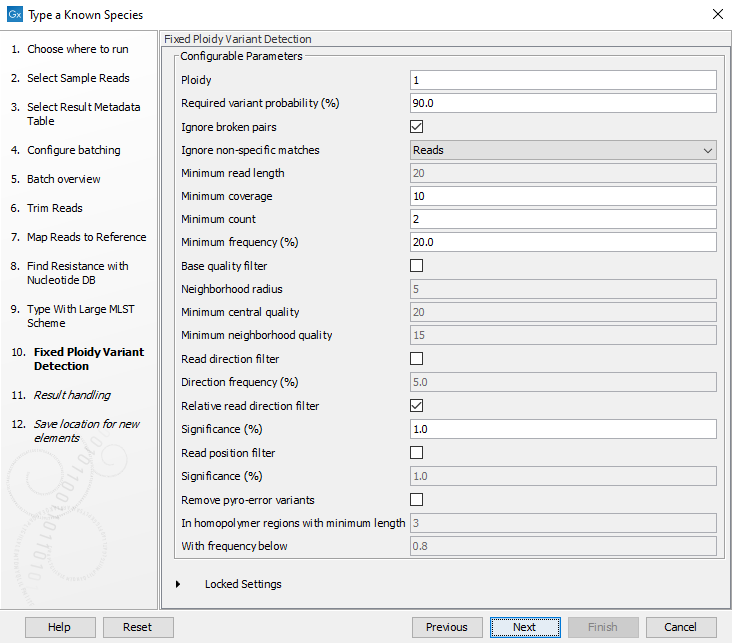
Figure 10.32: Specify the parameters to be used for the Fixed Ploidy Variant Detection tool.The parameters that can be set are:
- Ploidy: The expected ploidy of the species of interest.
- Ignore broken pairs: You can choose to ignore broken pairs by clicking this option.
- Ignore non-specific matches: You can choose to ignore non-specific matches between reads, regions or to not ignore them at all.
- Minimum read length: Only variants in reads longer than this size are called.
- Minimum coverage: Only variants in regions covered by at least this many reads are called.
- Minimum count: Only variants that are present in at least this many reads are called.
- Minimum frequency %: Only variants that are present at least at the specified frequency (calculated as count/coverage) are called.
- Base quality filter: The base quality filter can be used to ignore the reads whose nucleotide at the potential variant position is of dubious quality.
- Neighborhood radius: Determine how far away from the current variant the quality assessment should extend.
- Minimum central quality: Reads whose central base has a quality below the specified value will be ignored. This parameter does not apply to deletions since there is no "central base" in these cases.
- Minimum neighborhood quality: Reads for which the minimum quality of the bases is below the specified value will be ignored.
- Read direction filters: The read direction filter removes variants that are almost exclusively present in either forward or reverse reads.
- Direction frequency %: Variants that are not supported by at least this frequency of reads from each direction are removed.
- Relative read direction filter: The relative read direction filter attempts to do the same thing as the Read direction filter, but does this in a statistical, rather than absolute, sense: it tests whether the distribution among forward and reverse reads of the variant carrying reads is different from that of the total set of reads covering the site. The statistical, rather than absolute, approach makes the filter less stringent.
- Significance %: Variants whose read direction distribution is significantly different from the expected with a test at this level, are removed. The lower you set the significance cut-off, the fewer variants will be filtered out.
- Read position filter: This filter removes variants that are located differently in the reads carrying it than would be expected given the general location of the reads covering the variant site.
- Significance %: Variants whose read position distribution is significantly different from the expected with a test at this level, are removed. The lower you set the significance cut-off, the fewer variants will be filtered out.
- Remove pyro-error variants: This filter can be used to remove insertions and deletions in the reads that are likely to be due to pyro-like errors in homopolymer regions. There are two parameters that must be specified for this filter:
- In homopolymer regions with minimum length: Only insertion or deletion variants in homopolymer regions of at least this length will be removed.
- With frequency below: Only insertion or deletion variants whose frequency (ignoring all non-reference and non-homopolymer variant reads) is lower than this threshold will be removed.
Click Next.
- In the Result handling window, pressing the button Preview All Parameters allows you to preview - but not change - all parameters. Choose to save the results (we recommend to create a new folder for it) and click Finish.
The output will be saved in the new folder you created (figure 10.33), but those marked with a (*) in the list below will also be added automatically to the Metadata Result table.
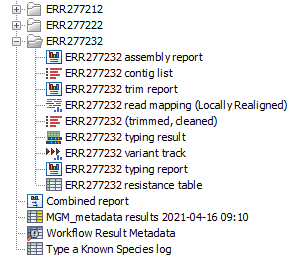
Figure 10.33: Output files from the Type a Known Species workflow.
- Trim report: report from the Trim Sequences tool (see http://resources.qiagenbioinformatics.com/manuals/clcgenomicsworkbench/current/index.php?manual=Trim_output.html).
- Read mapping: output from the Local Realignment tool, mapping of the reads to the specified reference.
- Trimmed, cleaned sequences: list of the sequences that were successfully trimmed and mapped to the best reference.
- Assembly summary report: see http://resources.qiagenbioinformatics.com/manuals/clcgenomicsworkbench/current/index.php?manual=De_novo_assembly_report.html.
- Contig list: contig list from the De novo assembly tool.
- (*)Contig list resistance table: result table from the Find Resistance with Nucleotide DB tool, reports the found resistance.
- (*)Typing report: output from the Type with Large MLST Scheme tool, includes information on the applied MLST scheme, the best matching sequence type (ST) as well as an overview table with sample information and a table summarizing the allele calls.
- Typing result: output from the Type with Large MLST Scheme tool, includes information on kmer fractions, kmer hit counts and allele count, identified and called.
- Variant Track: output from the Fixed Ploidy Variant Detection tool. Note that it is possible to export multiple variant track files from monoploid data into a single VCF file with the Multi-VCF exporter.This exporter becomes available when installing the CLC Microbial Genomics Module. All variant track files must have the same reference genome for the Multi-VCF export to work.
For each batch analysis run, the following outputs are generated:
- Combined report: combines the information from the trim report and MLST typing report.
- Results metadata table: An table containing summary information for each sample analyzed and a quick way to find the associated files. In addition, an extra column in the Result Metadata Table called "Best match, average coverage" helps the user to decide if a best match is significant, well covered and of good quality. This is especially helpful when a sample has low quality but is not contaminated.
