SignalP background
The importance of signal peptides was shown in 1999 when Günter Blobel received the Nobel Prize in physiology or medicine for his discovery that "proteins have intrinsic signals that govern their transport and localization in the cell" [Blobel, 2000]. He pointed out the importance of defined peptide motifs for targeting proteins to their site of function.
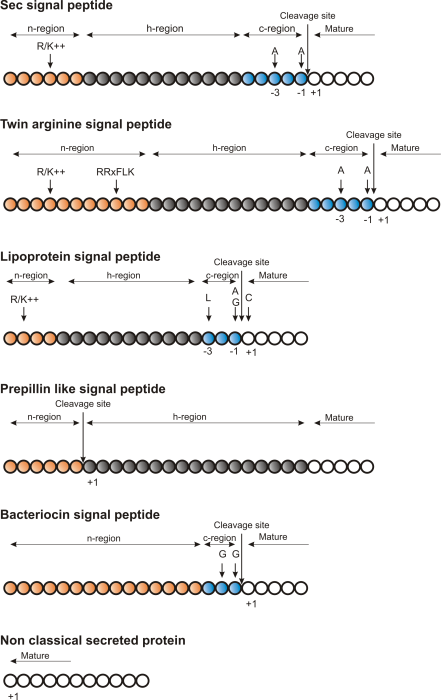
Figure 2.5: Schematic representation of various signal
peptides. Red color indicates n-region, gray color indicates
h-region, cyan indicates c-region. All white circles are part of the
mature protein. +1 indicates the first position of the mature
protein. The length of the signal peptides is not drawn to scale.
Soon after Günter Blobel's initial discovery of signal peptides, more targeting signals were found. Most cell types and organisms employ several ways of targeting proteins to the extracellular environment or subcellular locations. Most of the proteins targeted for the extracellular space or subcellular locations carry specific sequence motifs (signal peptides) characterizing the type of secretion/targeting it undergoes.
Targeting motifs can either be removed from, or retained in the mature protein after the protein has reached the correct and final destination. Some of the best characterized signal peptides are depicted in figure 2.5.
